Why Salt Fish Don't Survive In Fresh Water?
Why Salt fish don’t survive in fresh water?A marine fish’s chances of surviving are reduced when it is kept in a freshwater tank. This is a result of their bodies adaptation to the extreme salinity found in the maritime environment. They are unable to control the amount of water that enters their bodies (via osmosis) in freshwater environments. A marine fish would begin absorbing large amounts of water through its gills if it were placed in fresh water. It wouldn’t take long for its body’s water and salt balance to become out of balance. It may even enlarge! This is because they absorb water by osmosis because their bodies are more salted than fresh water. It is not intended for the aquariums themselves to be used as freshwater or saltwater tanks. As a result, if you wish to switch up the type of system, you can utilize the same tank. Freshwater aquatic life, however, cannot survive in a salty environment. Fish classified as euryhaline species are those that, at some point in their life cycle, can withstand a broad range of salinity. These species can live or survive in a wide range of salinities, from fresh to brackish to marine waters. They include salmon, eels, red drum, striped bass, and flounder. This is because they absorb water by osmosis because their bodies are more salted than fresh water. Freshwater fish experience the same issue, but they have evolved to quickly eliminate water, primarily by frequent urination. Some fish, such as salmon and eels, may transition from living in freshwater to the sea by changing their biology, although this process requires a lot of energy and time.
WHAT IS OSMOSIS?

Osmosis You must first comprehend the osmosis process in order to comprehend why freshwater fish cannot survive in the ocean and saltwater fish cannot survive in freshwater. Osmosis is the process by which liquid molecules travel from a low concentrated solute to a high concentrated solute across a semipermeable membrane (such as the thin layer inside an egg). A semipermeable membrane is a barrier with tiny holes that allow liquid molecules to pass through while obstructing the passage of concentrated substances like sugar or salt. Because a cell’s membrane is semipermeable, smaller molecules, like water molecules, can get through while larger ones are blocked. A raisin, for instance, will swell up if you try to submerge it in fresh water because the raisin has a higher sugar concentration than the freshwater and the water will keep coming in. However, a raisin will shrivel much more in supersaturated salt water as the water will drain out of it. Compared to raisins, the supersaturated salt water has a higher concentration of content.
OSMOTIC PRESSURE AND TONICITY
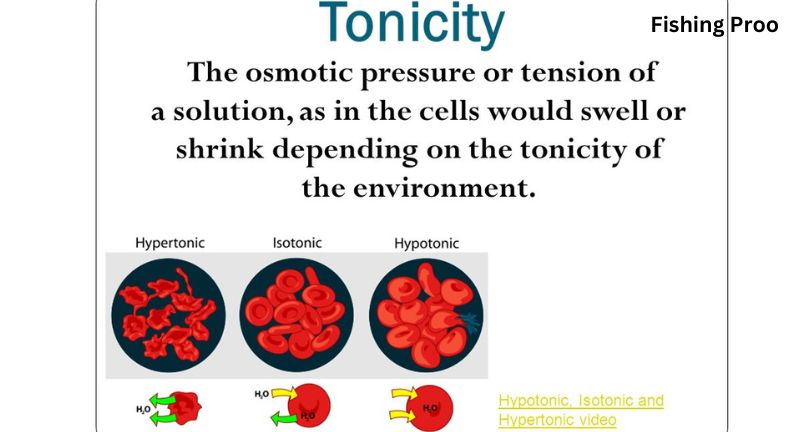
The pressure that is provided to prevent water molecules from passing through a semipermeable membrane is known as osmotic pressure. The osmotic pressure tonicity when water flows through a semipermeable membrane and into a more concentrated solution. Water does not required to cross a membrane if the concentration of the solution is the same on both sides. Consequently, there is no osmotic pressure.
Osmotic pressure is measured in terms of tonicity. To gain a better understanding of why fish can only live in particular types of water, you need be aware of the three different classes of tonicity. There are three categories for tonicity:
- Overtonity
- The state of being in equilibrium
- Inadequate Tonality
Why salt fish don't survive in fresh water
Why Salt fish don’t survive in fresh water? Certain fish species, meanwhile, are able to live in both freshwater and saltwater. The fish species, known as euryhaline fish, can live in either body of water for a set period of time and can even migrate there. Anadromous and catadromous euryhaline fish are the two different varieties. Though they are born in freshwater, anadromous fish live their whole lives in the ocean, coming back only to spawn. Salmon, smelt, striped bass, and sturgeon are a few of these fish species. On the other hand, freshwater fish are known as catadromous fish since they must cross rivers to reach the sea in order to breed. The European Eel is one species of fish that is a catadromous.
Handling Sulfur: Osmoregulation
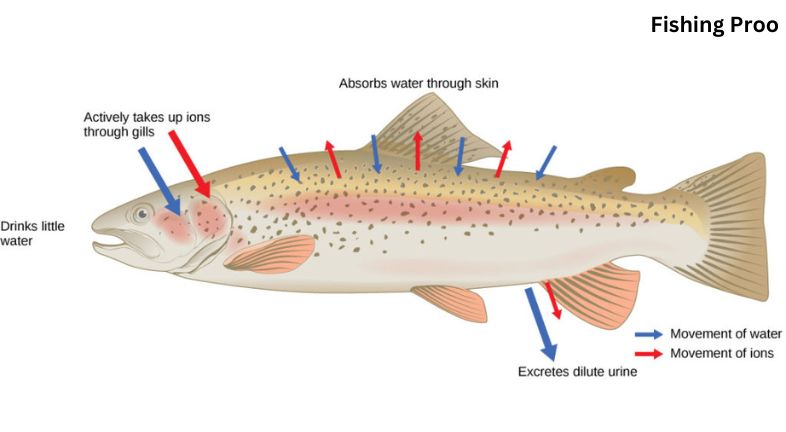
Handling sulfur,Water is the foundation of all life. Because of this, all organic material that makes up humans floats or comes into contact with water. Life, however, needs to preserve a vital balance between salt and water. Life isn’t happy (or alive) when there is too much or too little of either. Osmoregulation, or the regulation of osmosis, is the mechanism by which living organisms balance their water requirements. Water moves from a solution with a lower concentration to one with a higher concentration across a semipermeable membrane through a process known as osmosis. To put it simply, osmosis is the process by which water passes through a semi-permeable membrane—a membrane that will only allow water or similarly sized molecules to pass through it. Hopefully, this picture will make it easier to understand.
CONCLUSION:
Fresh water floats above seawater because it is less dense than saltwater. Between the two water bodies, a distinct border is formed, with fresh water floating on top and a wedge of saltwater on the bottom. At the boundary between the two water masses, there is some mixing, but it’s usually very little. A marine fish’s chances of surviving are reduced when it is kept in a freshwater tank. This is a result of their bodies adaptation to the extreme salinity found in the maritime environment. They are unable to control the amount of water that enters their bodies (via osmosis) in freshwater
environments.
Because the ocean is so salty, the concentration of water inside fish is higher than that of the
ocean. Thus, the majority of saltwater fish consistently lose water through their gills and skin.








1 thought on “Why Salt Fish Don’t Survive In Fresh Water?”
This is really interesting, You are a very skilled blogger. I have joined your feed and look forward to seeking more of your magnificent post. Also, I’ve shared your site in my social networks!